InsightRX Nova Precision Dosing in Clinical Trials
Maximize Clinical Trial Success with MIPD
Empower your biopharma company to integrate precision dosing into clinical trials with a quality-compliant, end-to-end dosing platform.
InsightRX Nova
Core features and capabilities
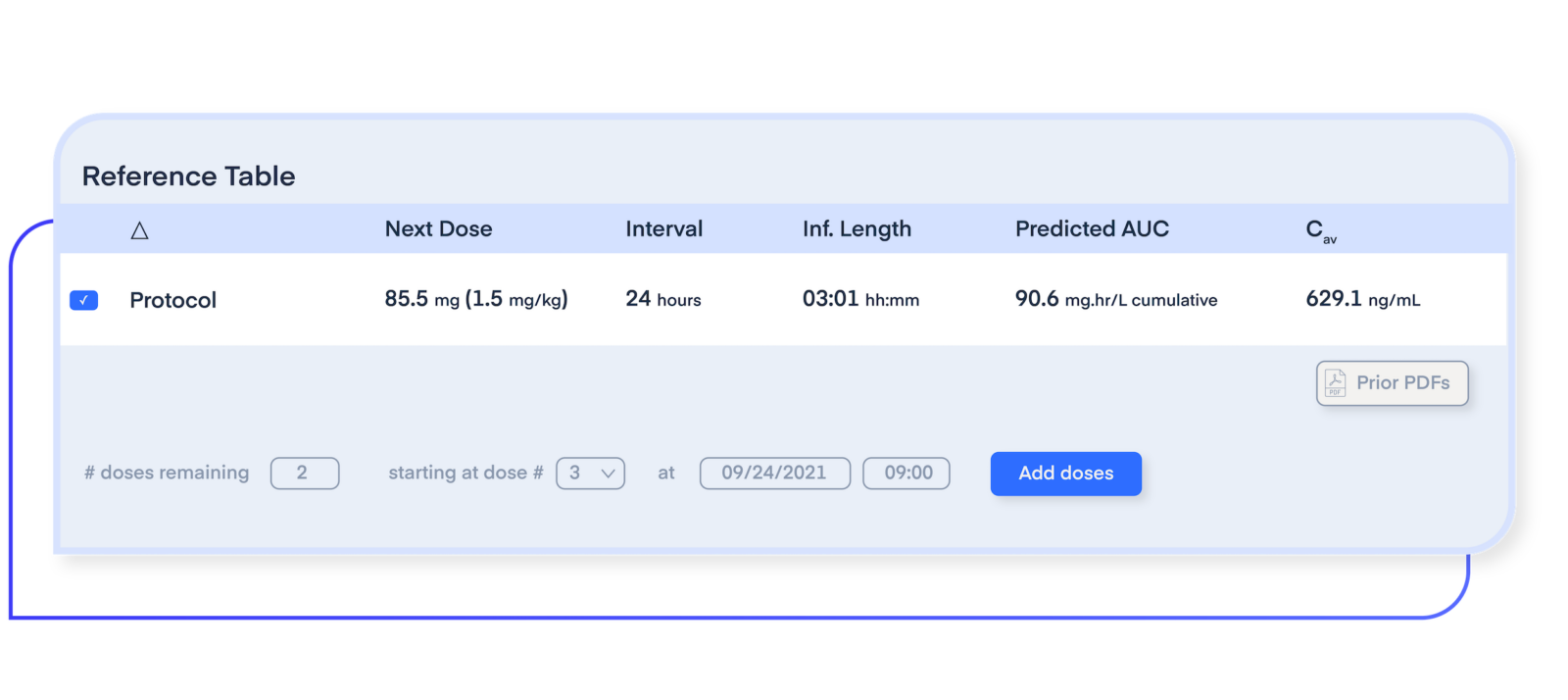

Increase program success with precision dosing intelligence
Plan and execute drug optimization strategies starting in Phase I. Leverage model-informed precision dosing (MIPD), Bayesian forecasting, and machine learning to test multiple dose ranges for easy identification of target dose levels and intervals.
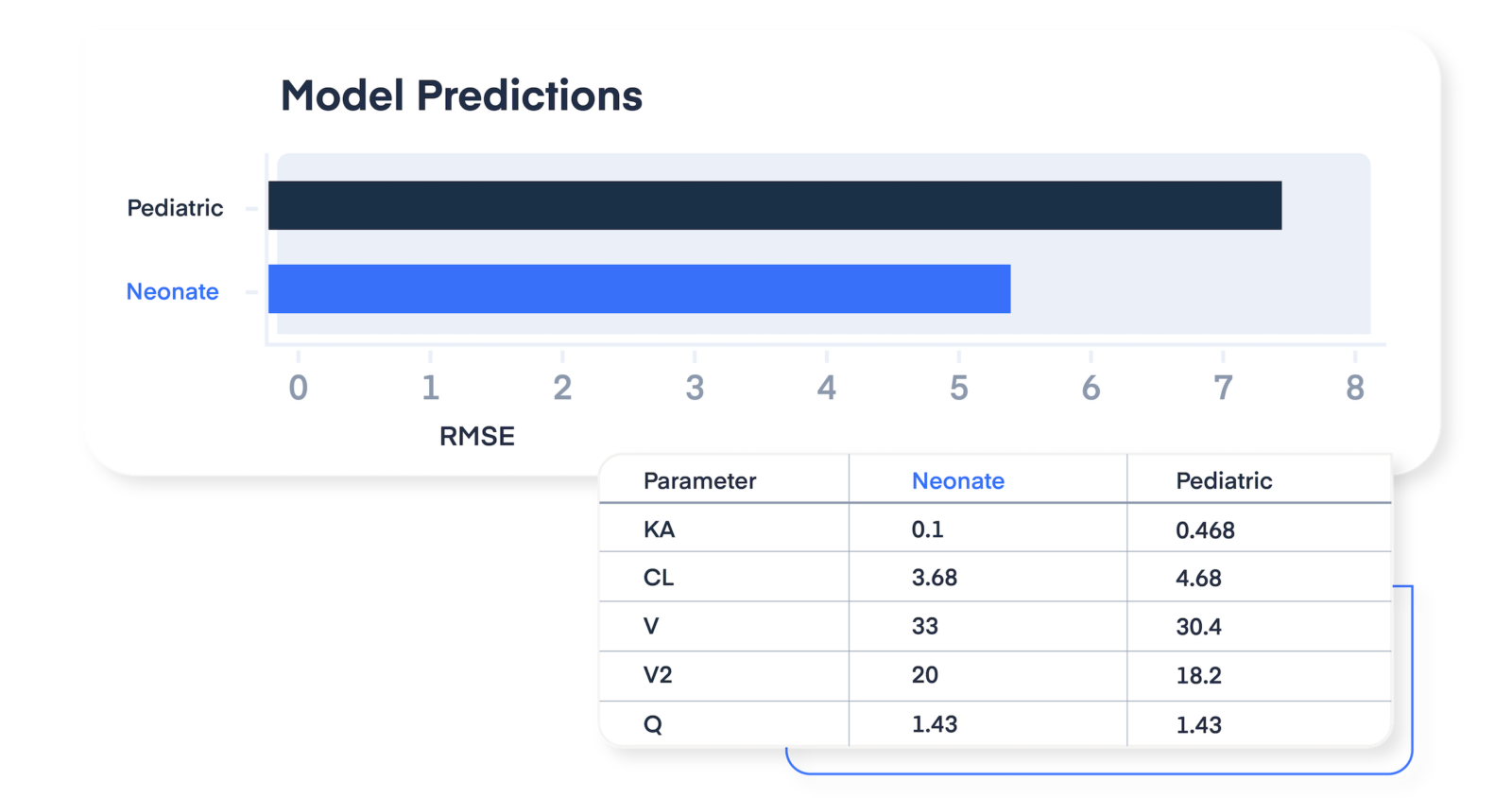
Maximize drug efficacy and safety for participants
Avoid sub-therapeutic and supra-therapeutic dosing by targeting specific drug exposures or biomarker ranges. InsightRX Nova accounts for individual concentration levels, lab results, and patient demographics — helping to sidestep risks associated with maximum tolerated dose (MTD)-based regimens.


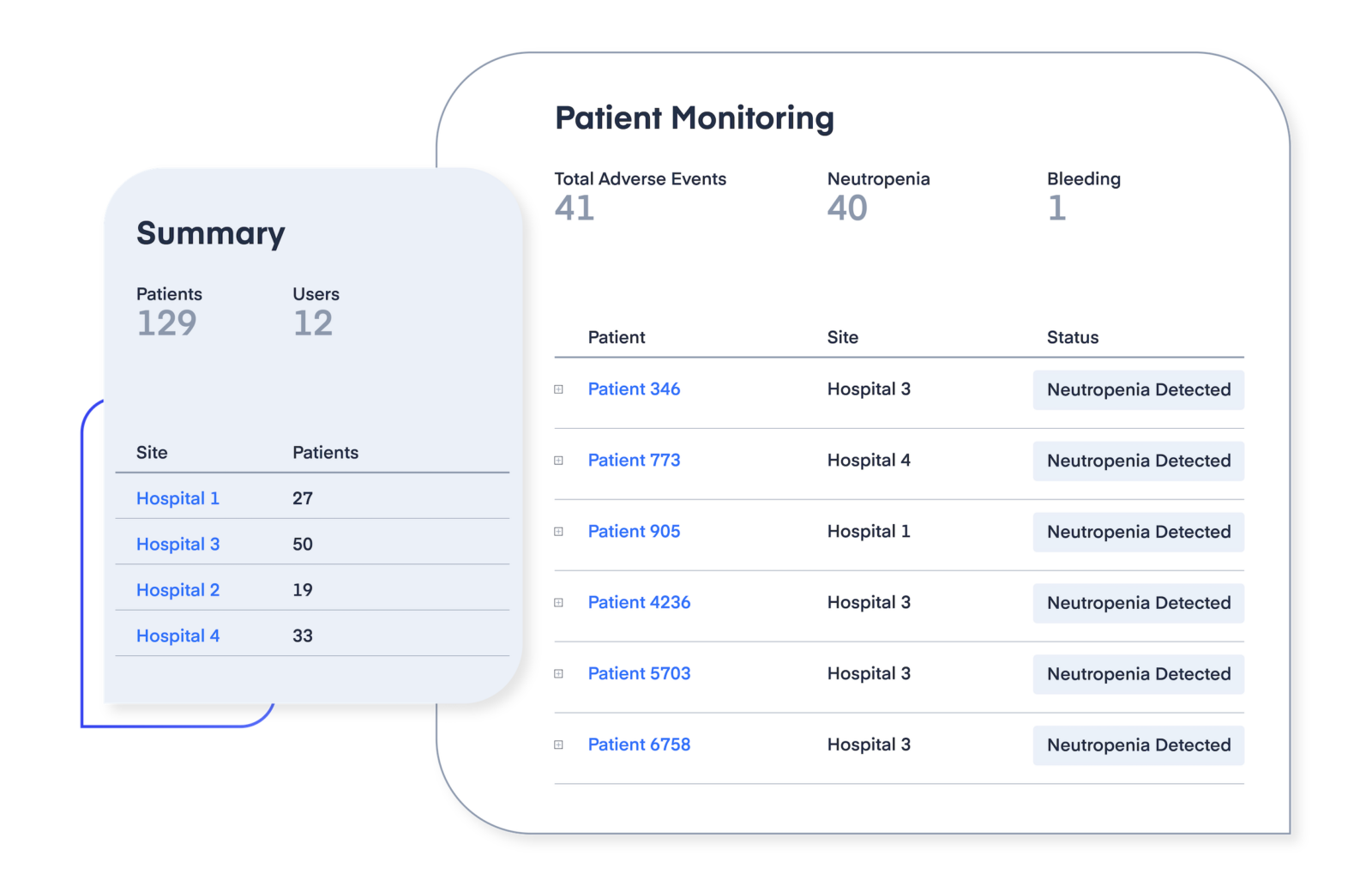
Boost patient enrollment and retention in trials
High variability and adverse drug reactions can drive patient dropout, costing organizers up to 30% of enrollees and significant financial impact.
Precision dosing supports therapeutic target achievement and uses real-time drug response predictions to identify vulnerable patients early—reducing adverse events and improving retention.
Additional Information
Learn more about our products and services:
Explore Advanced PK/PD Analytics
Complement your precision dosing strategy with powerful pharmacokinetic and pharmacodynamic analytics. InsightRX Apollo enables deeper insights into drug behavior across populations to optimize dosing and support adaptive trial designs.
Enhance Decisions with Companion Apps
Use InsightRX companion apps to deliver real-time, user-friendly dosing guidance. These tools integrate seamlessly with trial workflows to improve protocol adherence and support precise therapy management.






