InsightRX Apollo PKPD Analytics for Clinical Trials
Drive Smarter Clinical Trials Through Advanced PKPD Visualization
- Home
- MIPD for Life Sciences
- InsightRX Apollo for Life Sciences
Deliver advanced PKPD analytics to empower biopharma teams with deep data insights
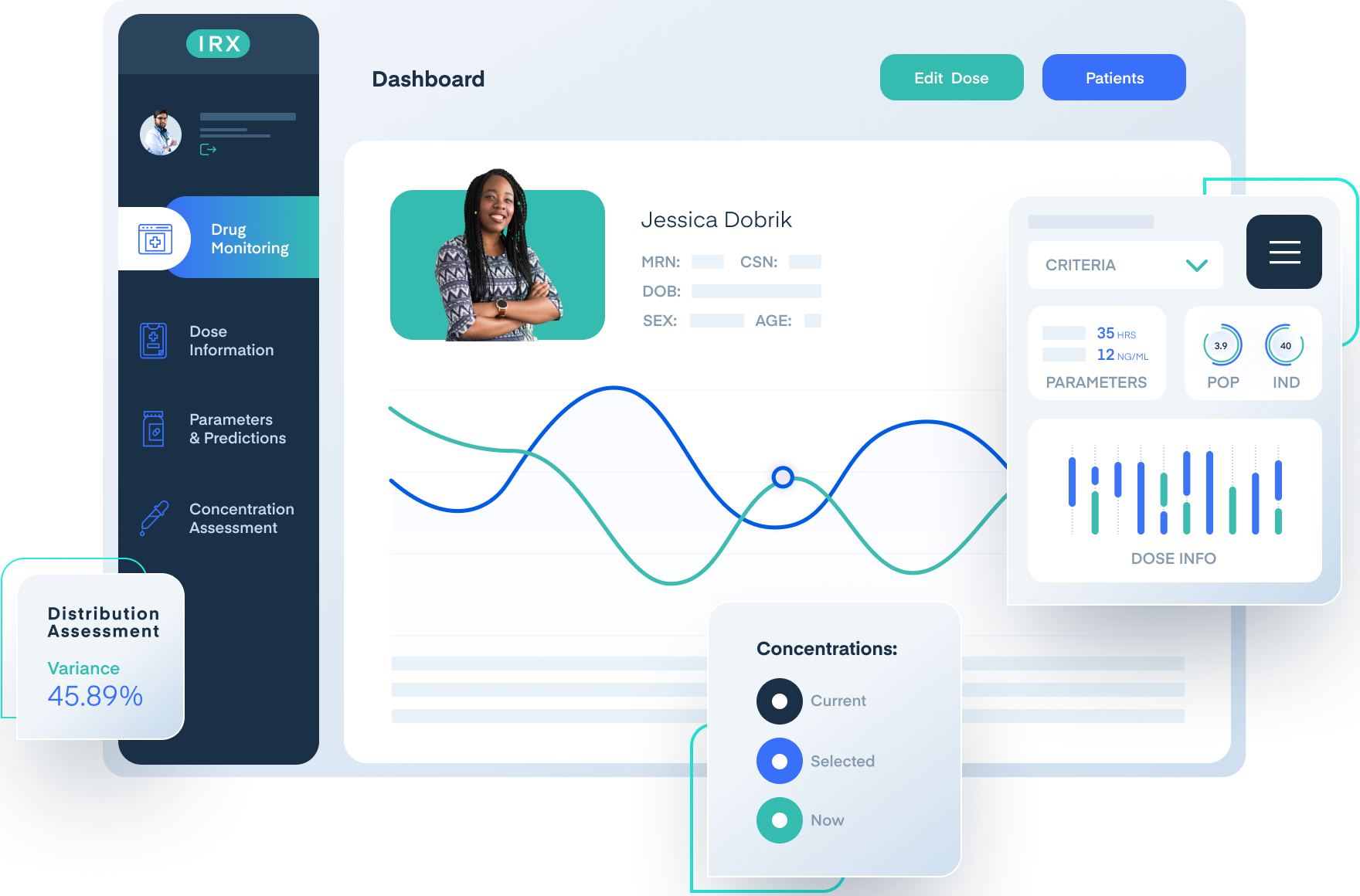
Comprehensive PK/PD visualization
InsightRX Apollo provides robust tools to analyze and visualize PK/PD data across diverse patient populations.
Comprehensive analytics enable biopharma teams to detect trends, identify outliers, and define therapeutic windows, supporting data-driven dosing decisions.

Support for adaptive and precision dosing trials
The platform integrates seamlessly into clinical trial workflows to enable dynamic dose adjustments and adaptive study designs.
Help your teams improve trial efficiency, patient safety, and regulatory confidence.

Streamlined regulatory submission support
Generate detailed, audit-ready reports that comply with regulatory requirements.
With InsightRX Apollo, facilitate smoother submissions, faster approvals, and maintain full data integrity throughout the trial lifecycle.

Additional Information
Learn more about our products and services:
Drive Success in Trials with Precision Dosing
Leverage InsightRX’s model-informed precision dosing platform to individualize dosing regimens, reduce variability, and improve safety and efficacy throughout your clinical development program.
Enhance Decisions with Companion Apps
Use InsightRX companion apps to deliver real-time, user-friendly dosing guidance. These tools integrate seamlessly with trial workflows to improve protocol adherence and support precise therapy management.






